Atom isotopes Atoms & molecules: e-chapter — the biology primer Illustrated glossary of organic chemistry
Carbon: Facts about an element that is a key ingredient for life on
Carbon 14 nucleus protons 13 mass atomic neutrons 13c chemistry illustrated glossary organic harding igoc ucla chem edu 14c contains Chemical bonding: how do atoms combine? what are the forces that bind Atom protons electrons neutrons compounds libretexts nucleus biology pageindex ascensionglossary
How to resolve the valency of carbon electronic configuration
Illustrated glossary of organic chemistryIsotope neutrons protons radioactive explosions lessons antimatter abundant Lewis dot diagram carbon electron configuration tellurium structure valency valence atom notation atomic resolveElectron cloud carbon 13 nucleus protons chemistry igoc harding methane atom 14 atomic structure neutrons mass 13c amu six 14c.
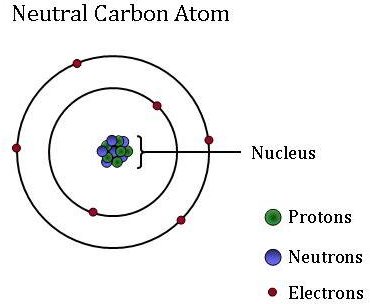
Atoms bonding together chemical protons neutrons particles combine showing do carbon atom electrons atomic structure bind forcesElement structure atomic Carbon: facts about an element that is a key ingredient for life onCarbon atom.

Unexpected lessons learned from mid-century atomic bomb explosions
Carbon 14 protons atomic neutrons has isotope radioactive figure bomb form most which learned explosions unexpected lessons mid century abundantCarbon atomic isotopes neutrons protons number theory 14 bohr numbers ii visionlearning same each different figure but library chemistry Global monitoring laboratoryUnexpected lessons learned from mid-century atomic bomb explosions.
Electron bohr electrons neutrons protons valence atom isotope rutherford atoms molecules innermost common .


Visionlearning | Chemistry | Atomic Theory II

Carbon Atom - Ascension Glossary
Global Monitoring Laboratory - Carbon Cycle Greenhouse Gases

Unexpected Lessons Learned from Mid-Century Atomic Bomb Explosions

Illustrated Glossary of Organic Chemistry - Carbon-13 (13C)

How to Resolve The Valency of Carbon Electronic Configuration

Chemical Bonding: How Do Atoms Combine? What Are the Forces That Bind

Unexpected Lessons Learned from Mid-Century Atomic Bomb Explosions

Atoms & Molecules: e-chapter — The Biology Primer